- Schedule 3 Non Narcotics List
- Schedule I, II, III, IV, & V Drugs | Drug Classifications ...
- Class 3 Narcotic List
- Amobarbital
- List Of Schedule 3 Narcotics
- Chlorhexadol
39-17-410. Controlled substances in Schedule III.
Examples of Schedule III narcotics include: products containing not more than 90 milligrams of codeine per dosage unit (Tylenol with Codeine®), and buprenorphine (Suboxone®). Examples of Schedule IIIN non-narcotics include: benzphetamine (Didrex®), phendimetrazine, ketamine, and anabolic steroids such as Depo®-Testosterone. Part 4 - Drugs 39-17-410 - Controlled substances in Schedule III. Controlled substances in Schedule III. (a) Schedule III consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section.
(a) Schedule III consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section.
(b) Stimulants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following substances having a stimulant effect on the central nervous system, including its salts, isomers (whether optical, positional or geometric), and salts of such isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation:
(1) Those compounds, mixtures, or preparations in dosage unit form containing any stimulant substances listed in Schedule II, which compounds, mixtures, or preparations were listed on August 25, 1971, as excepted compounds under 21 CFR 1308.32, and any other drug of the quantitative composition shown in that list for those drugs or that is the same except that it contains a lesser quantity of controlled substances;
(2) Benzphetamine;
(3) Chlorphentermine;
(4) Clortermine; and
(5) Phendimetrazine.
(c) Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following substances having a depressant effect on the central nervous system:
(1) Any compound, mixture, or preparation containing:
(A) Amobarbital;
(B) Secobarbital;
(C) Pentobarbital;
or any salt thereof and one (1) or more other active medicinal ingredients that are not listed in any schedule;
(2) Any suppository dosage form containing:
(A) Amobarbital;
(B) Secobarbital;
(C) Pentobarbital;
or any salt of any of these drugs and approved by the United States food and drug administration for marketing only as a suppository;
(3) Any substance that contains any quantity of a derivative of barbituric acid or any salt thereof;
(4) Chlorhexadol;
(5) [Deleted by 2007 amendment.]
(6) Lysergic acid;
(7) Lysergic acid amide;
(8) Methyprylon;
(9) Sulfondiethylmethane;
(10) Sulfonethylmethane; or
(11) Sulfonmethane;
(12) Any drug product containing gamma hydroxybutyric acid, including its salts, isomers, and salts of isomers, for which an application of § 505 of the federal Food, Drug, and Cosmetic Act, codified in 21 U.S.C. § 355;
(13) Tiletamine and zolazepam or any salt of tiletamine or zolazepam;
(A) Another trade or other name for a tiletamine-zolazepam combination product: Telazol;
(B) Another trade or other name for tiletamineis 2-(ethylamino)-2-(2-thienyl)-cyclohexanone.
(C) Other trade or other names for zolazepam are 4-(2-fluorophenyl)-6,8- dihydro-1,3,8-trimethylpyrazolo-[3,4-e], [1,4]-diazepin-7(1H)-one and flupyrazapon.
(d) Nalorphine.
(e) Narcotic Drugs.
(1) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities:
(A) Not more than one and eight tenths (1.8) grams of codeine per one hundred (100) milliliters or not more than ninety (90) milligrams per dosage unit, with an equal or greater quantity of an isoquinoline alkaloid of opium;
(B) Not more than one and eight tenths (1.8) grams of codeine per one hundred (100) milliliters or not more than ninety (90) milligrams per dosage unit, with one (1) or more active, nonnarcotic ingredients in recognized therapeutic amounts;
(C) Not more than three hundred (300) milligrams of dihydrocodeinone per one hundred (100) milliliters or not more than fifteen (15) milligrams per dosage unit, with a fourfold or greater quantity of an isoquinoline alkaloid of opium;
(D) Not more than three hundred (300) milligrams of dihydrocodeinone per one hundred (100) milliliters or not more than fifteen (15) milligrams per dosage unit, with one (1) or more active nonnarcotic ingredients in recognized therapeutic amounts;
(E) Not more than one and eight tenths (1.8) grams of dihydrocodeine per one hundred (100) milliliters or not more than ninety (90) milligrams per dosage unit, with one (1) or more active nonnarcotic ingredients in recognized therapeutic amounts;
(F) Not more than three hundred (300) milligrams of ethylmorphine per one hundred (100) milliliters or not more than fifteen (15) milligrams per dosage unit with one (1) or more active nonnarcotic ingredients in recognized therapeutic amounts;
(G) Not more than five hundred (500) milligrams of opium per one hundred (100) milliliters or per one hundred (100) grams or not more than twenty-five (25) milligrams per dosage unit, with one (1) or more active, nonnarcotic ingredients in recognized therapeutic amounts; or
(H) Not more than fifty (50) milligrams of morphine per ten (10) milliliters or per one hundred (100) grams, with one (1) or more active, nonnarcotic ingredients in recognized therapeutic amounts.
(2) Any material, compound, mixture, or preparation containing any of the following narcotic drugs or their salts: Buprenorphine.
(f) Anabolic steroids. Unless specifically excepted or unless listed in another schedule, any drug or hormonal substance, chemically and pharmacologically related to testosterone (other than estrogens, progestins, and corticosteroids) that promotes muscle growth, and includes:
(1) Boldenone;
(2) Chlorotestosterone;
(3) Clostebol;
(4) Dehydrochlormethyltestosterone;
(5) Dihydratestosterone;
(6) Drostanolone;
Schedule 3 Non Narcotics List
(7) Ethylestrenol;
(8) Fluoxymesterone;
(9) Formebulone;
Schedule I, II, III, IV, & V Drugs | Drug Classifications ...
(10) Mesterolone;
(11) Methandienone;
(12) Methandranone;
(13) Methandriol;
(14) Methandrostenolone;
(15) Methenolone;
(16) Methyltestosterone;
(17) Mibolerone;
(18) Nandrolone;
(19) Norethandrolone;
(20) Oxandrolone;
(21) Oxymesterone;
(22) Oxymetholone;
(23) Stanolone;
(24) Stanozolol;
(25) Testolactone;
Class 3 Narcotic List
(26) Testosterone;
(27) Trenbolone; and
(28) Any salt, ester, or isomer of a drug or substance described or listed in this subsection (f), if such salt, ester, or isomer promotes muscle growth.
(g) (1) Except as provided in subdivision (g)(2), subsection (f) shall not be construed to include an anabolic steroid that is expressly intended for administration through implants to cattle or other nonhuman species and that has been approved by the United States secretary of health and human services for such administration. Also, except as provided in subdivision (g)(2), subsection (f) does not include an anabolic steroid that is a combination of estrogen with anabolic steroid and that is expressly intended for administration to hormone deficient women.
(2) If any person prescribes, dispenses, or distributes a steroid described in subdivision (g)(1) for human use, that person shall be considered to have prescribed, dispensed, or distributed an anabolic steroid within the meaning of subsection (f). If any person prescribes, dispenses, or distributes an anabolic steroid that is a combination of estrogens with anabolic steroid and that is intended for administration to hormone deficient women, for use by persons who are not hormone deficient women, that person shall be considered to have prescribed, dispensed or distributed an anabolic steroid within the meaning of subsection (f).
(h) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of ketamine hydrochloride, including its salts:
Ketamine hydrochloride.
(i) Dronabinol (synthetic) in sesame oil and encapsulated in a soft gelatin capsule in a United States food and drug administration approved drug product.

[Acts 1989, ch. 591, § 1; 1992, ch. 700, § 3; 1996, ch. 694, §§ 1, 2; 2000, ch. 755, § 2; 2000, ch. 884, § 2; 2007, ch. 298, §§ 8-10.]
Call Anytime. We’re Here for You.
Treatment Support available 24/7
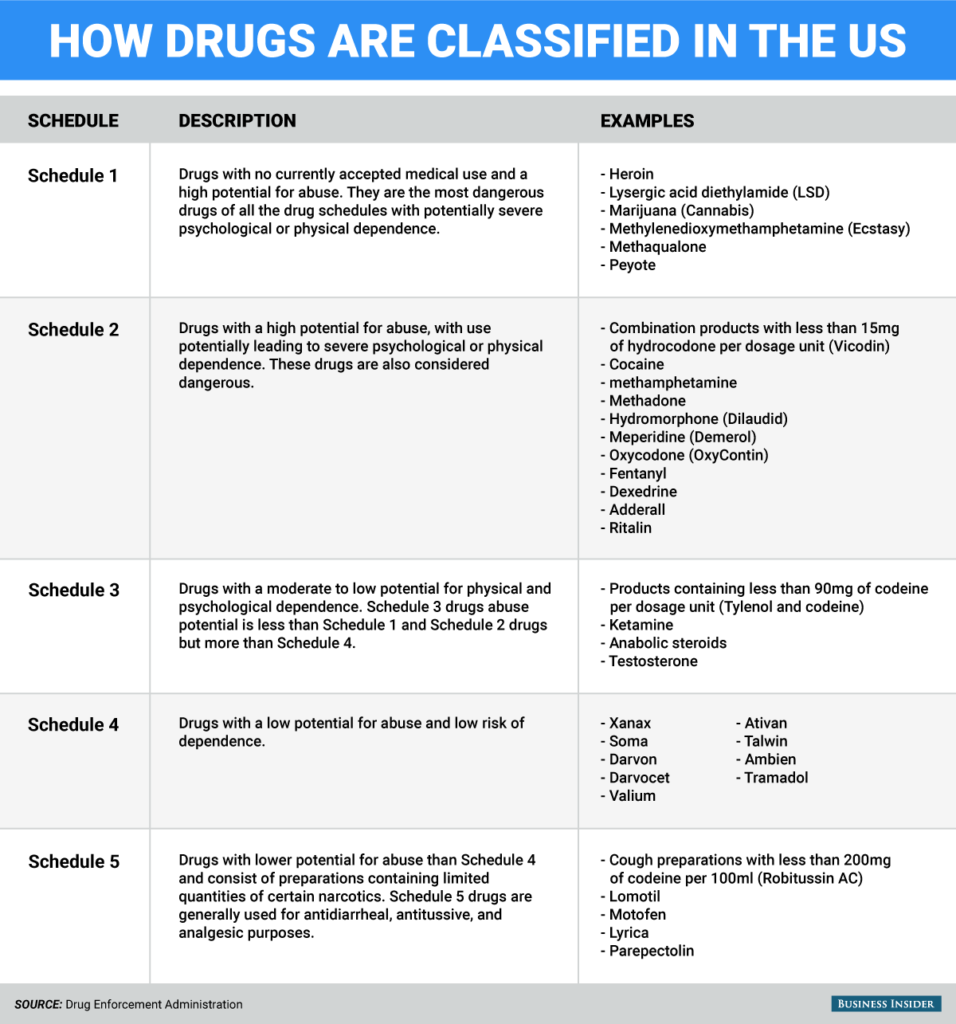
According to the Office of National Drug Control Policy, rates for unauthorized prescription drug use in 2009 nearly equaled rates for illegal drug use. In an effort to maintain some degree of control over potentially harmful drugs, the U.S. Drug Enforcement Administration issues guidelines for identifying and controlling certain types of drugs. As one of 5 different drug classes, the Schedule 3 narcotics list includes specific drugs that carry an identified level of risk for abuse.
U.S. Drug Policy
Vicodin and Tylenol with codeine are among the prescription drugs on the Schedule 3 list.
While lots of money flows through the illegal drug market, the market for licensed pharmaceuticals carries just as much potential for profit. Laws and regulations surrounding prescription drugs work to prevent illegal manufacturing and distribution practices. The Federal Food, Drug and Cosmetic Act of 1938 sets the parameters for which drugs fall within the “prescription drug” classification.
The five schedules or drug classes were put into place by the Controlled Substances Act of 1970. Each class of drugs carries a certain abuse potential that decreases with each successive class schedule. For example, Schedule 1 drugs hold the highest potential for abuse and dependency while the Schedule 5 drugs hold the lowest. The Schedule 3 narcotics list consists of drugs that hold a mild to moderate abuse potential. With the exception of Schedule 1 drugs, each class of drugs also holds certain accepted medical treatment uses.
Call Anytime. We’re Here for You.
(800) 407-7195Regulations surrounding controlled substances require authorized manufacturers and distributors to be licensed to handle the different drug classes with some classes requiring specialized licensing. Regulatory requirements extend to pharmacies, pharmacists as well as doctors and hospitals. As Schedule 3 narcotics list drugs carry a moderate risk of abuse, certain licensing requirements do apply for the people and agencies that handle these drugs.
Amobarbital
Schedule 3 Narcotics List
Each drug schedule follows certain guidelines concerning accepted medical uses, potential for abuse and potential for dependence. Drugs on the Schedule 3 narcotics list mainly consist of steroids, diet drugs and a few actual narcotic medications. This drug class includes both prescription and over-the-counter medications. No illegal or “street drugs” appear on the Schedule 3 narcotics list.
The Schedule 3 narcotics list includes the following drug types:
- Tylenol with Codeine
- Suboxone
- Didrex
- Depo-Testosterone
Vicodin and Tylenol with Codeine drugs must meet certain specifications in terms of ingredient amounts. Vicodin doses must contain no more than 15 milligrams of hydrocodone per unit. Tylenol with Codeine does must contain no more than 90 milligrams of codeine per unit.
Packaging and Distribution Requirements
Call Anytime. We’re Here for You.
Treatment Support available 24/7
Regulations regarding Schedule 3 narcotics list drugs provide guidelines for packaging that must be adhered to by manufacturers, practitioners and pharmacies. Prescriptions for Schedule 3 drugs can only be written by licensed medical practitioners, dentists, optometrists and veterinarians. All forms of drug packaging for Schedule 3 narcotics list drugs must display an “Rx-only” legend.
As each drug carries its own set of accepted and known medical uses, a drug can only be prescribed to treat certain corresponding conditions as listed under federal guidelines. Certain regulations also apply for over-the-counter Schedule 3 drugs in terms of types of ingredients and dosage amounts.
To learn more about Schedule 3 narcotics, or for help finding an addiction treatment program, call (800) 407-7195(Who Answers?) today!
List Of Schedule 3 Narcotics
the Take-Away
Chlorhexadol
Drugs on the Schedule 3 Narcotics List are all prescription medications that carry a certain level of abuse potential.



